After entering its tenth year with 19 rounds of negotiations, the concept of trade cooperation between Indonesia and the European Union or the Indonesia-European Union Comprehensive Economic Partnership Agreement (IEU-CEPA), has reached the final round. This year, Coordinating Minister for the Economy Airlangga Hartato said there will be an agreement signed.

On Sunday, July 13, 2025, President Prabowo Subianto met the President of the European Commission, Ursula von der Leyen, at the European Union Headquarters, Berlaymont Building, Brussels, Belgium.
During the meeting, both parties agreed to finalize the IEU-CEPA negotiations that have been ongoing for almost a decade. According to Airlangga, he has spoken with European Union Commissioner for Trade and Economic Security Maros Sefcovic, who plans to come to Jakarta in September 2025 to sign the agreement document.
"I have spoken with Commissioner Maros Sefcovic, and he plans to come to Jakarta in September to sign the document," he said. The government is targeting the IEU CEPA agreement to come into force in the fourth quarter of 2026 or at the latest in the first quarter of 2027.
The impact of IEU CEPA implementation will contribute to Indonesia's economic growth by 0.04 percent. This is the result of a cost and benefit study that has been conducted.

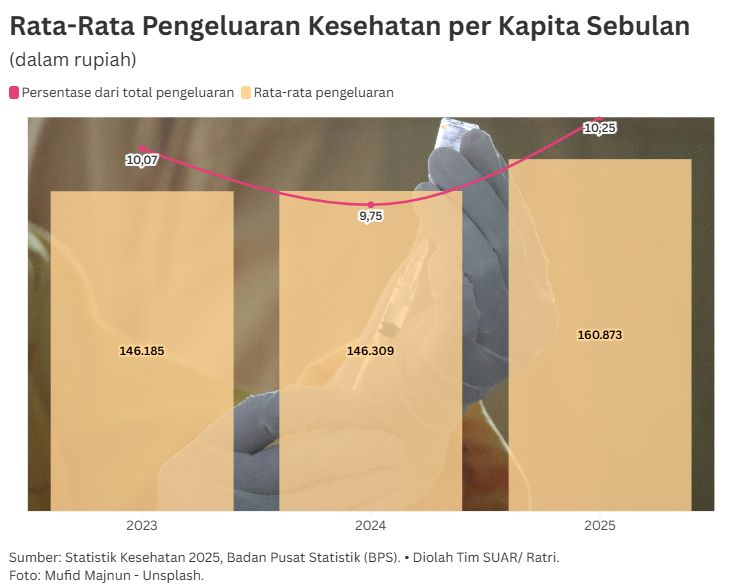
Europe's engine to boost healthcare
The long negotiation of Indonesia's cooperation with the European Union will include trade cooperation in the sectors of renewable energy, electric vehicles, information technology, pharmaceuticals, and medical devices. "The EU is very serious about increasing investment in these sectors," said Djatmiko B. Witjaksono, Director General of International Trade Negotiations of the Ministry of Trade, at Menara Kadin, August 4, 2025.
Djatmiko admitted that the government will open import taps for products from the European Union through IEU-CEPA. According to him, the move is expected to boost the performance of the national manufacturing sector. Import taps for the European Union will increase imports of high-tech machinery from Germany and France, which will indirectly increase the efficiency of domestic factories.
The government also hopes that the opening of import taps from the European Union, especially health machinery, can also improve the quality of health services in the country. Because, with many quality health machines from Europe in Indonesia, it will improve the quality of health services in the country.
"There are many technological medical equipment products from Europe that are needed in the country. We provide this facility so that health costs can be competitive in Indonesia," he said.
Regarding increasing the capacity of medical devices in Indonesia, the government has also begun to encourage investment from the European Union to build a medical device factory here. Minister Airlangga Hartarto said that one of the large local pharmaceutical companies, PT Kalbe Farma, is also exploring cooperation with European manufacturers.
One of the big local pharmaceutical companies, PT Kalbe Farma, is also exploring cooperation with European manufacturers.
"The most concrete investment for pharmaceutical medical equipment that has entered into cooperation is Kalbe (Farma), and we will make a factory in West Java," said Airlangga when met by reporters after attending the Second Quarter-2025 Economic Growth Press Conference in Jakarta on August 5, 2025.
Learning from the state of the drug industry
On paper, this cooperation will be beneficial for the domestic health industry. However, observers believe that without a long-term strategy, Indonesia could be trapped as a distribution point.
Currently, the entire activities of the domestic pharmaceutical industry still depend on imports of raw materials to the tune of 90%-95%, mostly from China and India. The 2020 pandemic crisis has proven that when supply is disrupted, chaos occurs.
"We should not only prepare the shelves, but also prepare the kitchen," said Heri Andreas, an economist from the Institute for Development of Economics and Finance (Indef), likening the risk. According to him, IEU-CEPA will only be beneficial in the long run if Indonesia negotiates conditions that bind investors to build research and raw material factories here.

He also suggested that before any investment from Europe comes in, local pharmaceutical companies should also be encouraged to build research centers before investors take over production functions. "Build the research center here first. If we can do good R&D here, they will be more interested in investing in the factory as well," he said.
The problem is that research is not cheap. The pharmaceutical industry allocates up to 15%-20% of revenue to research and development (R&D), with a high risk of failure. Plus, there is the challenge of strict patent protection in the intellectual property rights (IPR) chapter of the IEU-CEPA. Some clauses such as data exclusivity and patent linkage could make it difficult for local generic manufacturers to enter the market faster.
The ideal of a pharmaceutical regional hub
Meanwhile, the Indonesian Pharmaceutical Companies Association (GPFI) welcomes foreign investment with a strong caveat: without fair regulations, good intentions can undermine the independence of the domestic industry.
"If the European industry is interested in entering Indonesia, please go ahead, as long as it gets the same treatment as the local industry. Pharmaceutical requirements and conditions continue to increase, don't be lax," said GPFI Chairman, Tirto F. Kusnadi.
Secretary General of the Biopharmaceutical and Drug Raw Materials Association (AB3O), Irfat Hista Saputra, added that the independence of the Indonesian pharmaceutical industry is still constrained by the high dependence on imports of drug raw materials or Active Pharmaceutical Ingredients (API). "For pharmaceutical drug raw materials, the condition is still very dependent on imports. If it can be produced domestically, it will be very good," said Irfat.
Meanwhile, the production capacity of finished medicines is considered adequate. There are more than 200 drug factories operating in Indonesia, both owned by foreign investment (PMA) and local producers. PMA factories are generally members of the International Pharmaceutical Manufacturers Group (IPMG), while local producers are organized in the Indonesian Pharmaceutical Companies Association (GP Farmasi).

Irfat explained that AB3O and its administrators have compiled a study on incentives to encourage the development of the domestic medicinal raw material industry. The study has been submitted to a number of ministries and institutions, including the Ministry of Health, Ministry of Trade, Ministry of Industry, Food and Drug Administration (BPOM), and Coordinating Ministry for Maritime Affairs and Investment (Kemenko Marves).
"In general, the condition is good, we just need to improve it," he added.
If this strategy works, Indonesia will not only be able to reduce imports, but could become a regional hub for pharmaceutical production - utilizing agreements such as RCEP and APEC to penetrate the Asia Pacific market. S